Oestrogen Receptors
|
|
|
|
Ligand
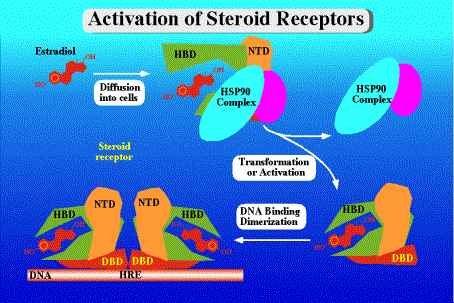
activation of ER mediates gene transcription. (NTD = N-teminal domain; DBD = DNA-binding domain ; HBD = hormne-binding domain) Picture courtesy of M Danielsen, the Nuclear Receptor Resource Library |
Cytoplasmic
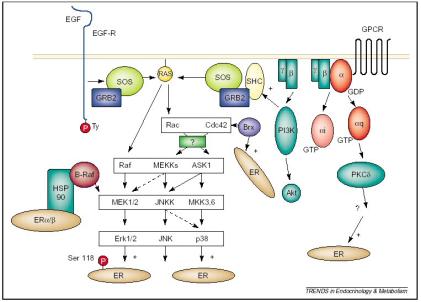
signalling pathways influencing the action of ER. Trends Endocrinol Metab. 13(10):422 (2002) P. H.
Driggers and J. H. Segars. Estrogen action and cytoplasmic signalling
pathways. |
How the oestrogen receptors work
In the absence of the oestrogen ligand, the ERs are found in the nucleus within a heat shock protein complex that inhibits their action. Upon binding to hormone, the ligand-binding domain changes shape to displace the heat shock proteins and to facilitate the binding of cofactors, which act to promote (coactivators) or inhibit (corepressors) the interaction of the receptor to its target genes. The ERs affect gene expression by either binding directly to DNA target genes through specific oestrogen response elements (ERE), or by binding to other DNA-bound transcription factors such as AP1, SP1 or NF-kappaB.
The ER is also capable of ligand-independent activity via a variety of intracellular signalling pathways, including the mitogen-activated protein kinase (MAPK) pathway. These signalling pathways exert their effects through the phosphorylation of the ER by protein kinases, or indirectly through the regulation of cofactors bound to the ER. The signal transduction effects involve non-transcriptional mechanisms, where cytoplasmic or cell membrane-bound regulatory proteins undergo steroid-induced changes. For instance, the action of ERa can be coupled to epidermal growth factor (EGF) signalling, where the activation of the EGF receptor leads to ERa phosphorylation via the MAPK pathway. The phosphorylation of ERa enhances its interaction with SRC (steroid receptor coactivator), and can affect ligand binding, receptor dimerisation and DNA binding. A feedback mechanism exists, where oestrogen can induce the activation of MAPK, which in turn activates the ER to amplify its signal. Other signalling proteins can also activate the ER in a ligand-independent way, including the cAMP-dependent PKA (protein kinase A) and PKC (protein kinase C). The cytoplasmic signalling pathways that effect oestrogen action produce various cell- and tissue-specific, oestrogenic responses through the assembly of distinct complexes. The ER is, therefore, able to act through several divergent pathways to bring about a variety of oestrogenic responses.
|
|
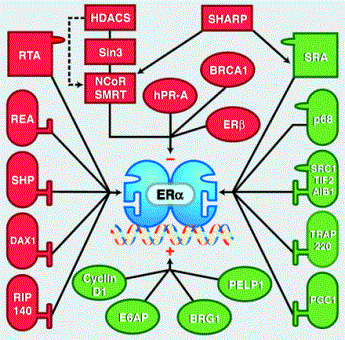
A
connection map for the human oestrogen receptor. The ER interacts with a large number of proteins that can
either positively or negatively regulate target gene transcription. Reprinted
from Science 296: 1642-1644 (2002) D. P. McDonnell and J. D. Norris. Connections
and Regulation of the Human Estrogen Receptor |
Cofactors effect oestrogen receptor action
ERs can interact with a variety of cofactors that modify ER action either by enhancing (coactivators) or inhibiting (corepressors) target gene transcription. Cofactors can bind to either the AF-1 site at the N-terminus, or to the AF-2 site in the ligand-binding domain. Coactivators function in histone acetylation to alter chromatin structure, and in the recruitment of RNA polymerase II to the promoter to enhance transcription. Corepressors function in histone deacetylation at the promoter to cause transcriptional repression, or they can act to block AF-2 activity on the ER itself to prevent coactivator binding. Cofactors can interact with several other nuclear receptors; as such, most cofactors are limiting factors, for which nuclear receptors compete with each other.
Several coactivators that bind to the AF-2 site in ERs have been identified, these include SRC, NCO2 (nuclear receptor coactivator 2), and NCO3 from the p160 family, which act by recruiting chromatin-modifying enzymes to the DNA-bound receptor; p300, CBP (CREB binding protein) and pCAF (CBP-associated factor) ), which function as histone acetylases for chromatin remodelling (may not bind ER directly); PPRB (PPAR binding protein), which functions to establish a link between the receptor and RNA polymerase II; and possibly NSD1, TF1A, PGC-1, and NCO6. Together these coactivators form a complex of proteins at target gene promoters to facilitate local decondensation of chromatin and the recruitment of the basal transcriptional machinery.
Coactivators can also bind to the AF-1 site, which is positively affected by MAPK-directed phosphorylation. The p160 family of proteins and CBP bind only weakly to AF-1, but other coactivators show a higher affinity for the AF-1 site, including the RNA coactivator SRA (steroid receptor RNA activator) and the RNA helicases p68 and p72.
Corepressors have been identified that bind to the AF-2 site; these include RIP140 (receptor-interacting protein), which antagonises SRC coactivators by competing for AF-2 binding; the orphan nuclear receptor SHP, which promotes corepressor deacetylase binding; the orphan nuclear receptor DAX-1, which acts as a dominant negative transcriptional regulator; NCR1 (nuclear receptor corepressor) and NCR2, which recruit histone deacetylases, and may mediate tamoxifen action in tumours; RBM9 (RNA-binding protein), which acts as a repressor of tamoxifen activity; REA (repressor of oestrogen action) and RSP5/RPF1.
Different ligands promote the binding of different cofactors. Ligands that act as agonists, such as estradiol-17b and diethylstilbestrol, can increase AF-2 activity to enhance coactivator binding, while those that act as antagonists, such as ICI and raloxifene, can block AF-2 activity to prevent coactivator binding, or can promote corepressor binding. Ligands can have different effects upon the two ERs, for instance THC can act as an ERa agonist and an ERb antagonist. Furthermore, ERa and ERb can interact with different affinities with various cofactors, which contributes to their differing responses. Various proteins can regulate cofactor activity, which in turn affects receptor binding and target gene activity; for instance, SRC activity can be increased by MAPK-mediated phosphorylation, and TF1A activity can be increased by the methyltransferase CARM1. The overexpression of various cofactors, such as NCO3, NCO6 and PPRB, has been found in both breast and ovarian cancers.
Next: The physiological roles of the oestrogen receptors
Previous: Oestrogen receptors,
ligand-inducible transcription factors