ATP Synthase
F-ATPases – How They Work
The F-ATPases can either produce ATP by harnessing the energy from a proton gradient, or they can work in reverse to create a gradient from the hydrolysis of ATP. F-ATPases provide a type of transporter for H+ ions to pass through the membrane, and possess a unique rotary motor that couples the flux of ions with the enzymatic synthesis or hydrolysis of ATP. Though the principle function of F-ATPases in mitochondria and chloroplasts appears to be ATP synthesis, in certain bacteria their main function seems to be the hydrolysis of ATP to create a gradient.
ATP Synthesis, the Movement of Ions
In order to synthesize ATP, F-ATPases must capture the energy from the flux of protons through the ATPase channel: a high concentration of H+ ions on one side of the membrane (created by electron transfer) causes the H+ ions to travel through the ATPase channel to the other side of the membrane where the H+ ion concentration is lower. The flux of H+ ions across the membrane drives the synthesis of ATP from ADP by F-ATPases.
Mitochondria contain two membranes: an outer membrane and a highly folded inner membrane, with a small inter-membrane space between them. The centre of mitochondria is called the matrix. H+ ions travel from the inter-membrane space to the mitochondrial matrix, because it is easier to accumulate a high concentration of ions in the small space between the two mitochondrial membranes than it is to fill the large central matrix, the later taking considerably more H+ ions to create a gradient. In addition, the inner membrane is highly folded to provide a greater surface area for ATP synthesis. The H+ gradient in mitochondria is produced during the tricarboxylic acid cycle in the breakdown of glucose, where H+ ions are stripped from the breakdown products of glucose and carried by NADH and FADH2 to the inter-membrane space. These H+ ions can only pass through the membrane via the ATP synthase channel, which harnesses this energy to make ATP.
By a similar process, the H+ ions in chloroplasts travel from the lumen through the ATPase channel in the thylakoid membrane to the stroma, and are supplied by the carrier NADPH, which in turn picks them up from the splitting of water using light energy (‘Photosystem II’). In bacteria, the H+ ions travel from the space between the plasma membrane and the cell wall (the periplasm in gram negative bacteria), through the ATPase channel in the plasma membrane to the cytoplasm, but the main function in some bacteria is ATP hydrolysis rather than synthesis.
F-ATPase Structure, a Unique
Rotary Motor
|
|
|
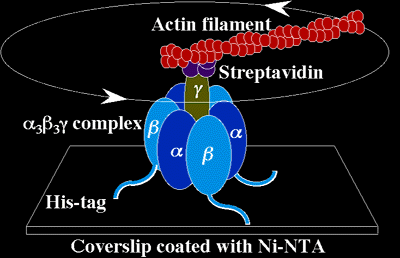
Diagram showing the rotary
motor of ATP synthase. Courtesy of Yoshida & Hisabori Lab |
F-ATPases are comprised of a soluble portion known as the F1 ATPase complex (enzyme activity), which consists five subunits (a, b, d, e and g), and a membrane-embedded portion known as the F0 ATPase complex (proton channel), which consists of at least three subunits (A, B and C) – in mitochondria, the F0 complex usually has 9 subunits (A-G, F6 and F8). There are some minor differences between the smaller subunits of F-ATPases found in bacteria, chloroplasts and mitochondria. The F1 ATPase complex is responsible for performing ATP synthesis or hydrolysis, while the F0 ATPase complex provides the proton channel for the translocation of H+ ions across the membrane. The F1 complex contains three a subunits, three b subunits, and one of each of the other subunits, where the three b-subunits are catalytic and the three a-subunits are regulatory in function. There is a substrate-binding site on each of the three a- and three b-subunits, but only those on the b-subunits are active sites, while those on the a-subunits are regulatory sites.
During catalysis by the F1 complex, some of the subunits rotate relative to the rest of the enzyme, making ATPase the smallest rotary motor known. In total, ATPase contains two rotational motors, one in F1 (g and e subunits) driven by ATP hydrolysis, the other in F0 (C subunit) driven by the H+ gradient, which are joined together so that the rotation of the two motors is coupled back-to-back. The two motors try to rotate in opposite directions, but the F0 motor is usually stronger, using the force from the H+ gradient flux to push the F1 rotary motor in reverse in order to drive ATP synthesis. Masasuke Yoshida has captured the amazing rotational motion of F-ATPase on video.
ATP hydrolysis
The reaction catalysed by F-ATPase is fully reversible, such that ATP hydrolysis can be used to create a H+ gradient by the reversal of the ion flux. In this case, the F1 rotary motor works in a forward motion to hydrolyse ATP, and to drive the F0 motor in reverse to create a H+ gradient. The generation of a H+ gradient can then be used to maintain ionic balance, as well as for active transport to drive substrate accumulation.
Next: What InterPro Tells Us
Previous: The
ATPase Family