Cholera Toxin
|
|
|
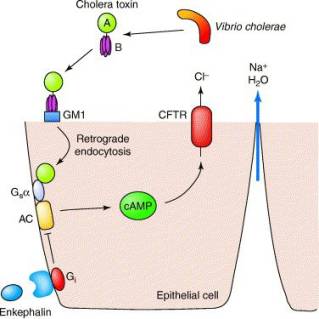
Action of cholera toxin. See below for description. A, B (cholera toxin subunits); GM1 (GM1 ganglioside receptor); Gsa (G protein); AC (adenylate cyclase); Gi (G protein); cAMP (cyclic AMP); CFTR (cystic fibrosis transmembrane conductance regulator). Reprinted
from Trends Pharmacol Sci. 26, J. R. Thiagarajah and A. S. Verkman, New
Drug Targets for Cholera Toxin, PP. 172-5, 2005, PMID:
15808339 |
The Actions of Cholera Toxin
When cholera toxin is released from the bacteria in the infected intestine, it binds to the intestinal cells known as enterocytes (epithelial cell in above diagram) through the interaction of the pentameric B subunit of the toxin with the GM1 ganglioside receptor on the intestinal cell, triggering endocytosis of the toxin. Next, the A/B cholera toxin must undergo cleavage of the A1 domain from the A2 domain in order for A1 to become an active enzyme. Once inside the enterocyte, the enzymatic A1 fragment of the toxin A subunit enters the cytosol, where it activates the G protein Gsa through an ADP-ribosylation reaction that acts to lock the G protein in its GTP-bound form, thereby continually stimulating adenylate cyclase to produce cAMP. The high cAMP levels activate the cystic fibrosis transmembrane conductance regulator (CFTR), causing a dramatic efflux of ions and water from infected enterocytes, leading to watery diarrhoea.
One area of anti-diarrhoea treatment lies in the stimulation of enkephalins, which regulate intestinal secretion by acting directly on enterocytes. Enkephalins bind to the opioid receptors on enterocytes, which act through G proteins to inhibit the stimulation of cAMP synthesis induced by cholera toxin, thereby directly controlling ion transport.
Diversity in Cholera Strains
The effects of cholera involve the actions of other Vibrio cholerae toxins that aid the pathogen in its colonisation, coordinated expression of virulence factors, and toxin action. These additional proteins include zona occludens toxin (zot, involved in Vibrio cholerae invasion by acting to decrease intestinal tissue resistance), accessory cholera toxin (ace, increases fluid secretion), toxin-coregulated pilus (tcpA, essential colonisation factor and receptor for the CTXf phage), NAG-specific heat-labile toxin (st), and outer membrane porin proteins (ompU and ompT). The expression of virulence factors is controlled by the transcriptional factors ToxR, TcpP and ToxT. Different strains of Vibrio cholerae produce differing sets and amounts of these auxiliary toxins, which in turn affect the clinical symptoms of cholera and its responsiveness to treatment.
For example,
the cholera outbreak in Russia in 1942 was caused by the El Tor biotype strain
of Vibrio cholerae, rather than the classical biotype that caused the pandemics
in the 19th and early 20th centuries. The El Tor biotype can carry several extra copies
of CTXf bacteriophage that contains the
toxin genes ctxAB (encodes cholera toxin A and B subunits), zot (encodes zona occludens
toxin) and ace (encodes accessory cholera toxin), leading to an increase in
cholera toxin production. The El Tor
biotype can also produce haemolysin, which is capable of lysing red blood cells
by attacking their membranes. In
addition, unlike the classical biotype, the El Tor biotype generates novel
toxin strains through CTXf phage
conversion. These El Tor strains
produce different, milder clinical symptoms, with many patients showing asymptomatic
cholera not accompanied by dehydration.
Next: What InterPro Tells Us
Previous: Cholera Toxin, the Main Culprit