Protein of the Month |
September 2006
MORE ON THIS MONTHíS PROTEIN
|
|
OTHER PROTEINS OF INTEREST |
|
Molecule of the Month: Elongation Factors |
|
|
Elongation Factors
By Jennifer McDowall
††††††††††† New proteins are in constant demand to accomplish the many varied tasks within a cell and to meet its ever-changing needs.† Ribosomes act to assemble proteins, accurately translating mRNA into polypeptide chains.† Ribosomes dominate the cytoplasm and can vary in number according to metabolic needs, a single cell containing hundreds or even thousands of ribosomes depending upon protein production levels.† In prokaryotic cells, ribosomes are free floating in the cytosol, whereas in eukaryotic cells they can be free floating or bound to the endoplasmic reticulum (ER).† Proteins made within the ER are often processed, exported or included in the cellís membranes.†
Ribosomes are complex macromolecular assemblies of protein and RNA (ribosomal RNA or rRNA).† The ribosomes of prokaryotic cells consist of three kinds of rRNA and about 50 types of proteins, while those of eukaryotic cells are more complex, consisting of five kinds of rRNA and about 80 kinds of proteins.† Ribosomes are composed of a large and a small subunit, which perform very different tasks.† The small subunit (30S in bacteria, 40S in eukaryotes) is responsible for binding the mRNA, interacting with the anticodon end of the tRNA to ensure the correct codon-anticodon interaction between mRNA and tRNA.† The large subunit (50S in bacteria, 60S in eukaryotes) is responsible for peptide bond formation in the growing peptide chain, interacting with the aminoacyl-bound end of the tRNA.†
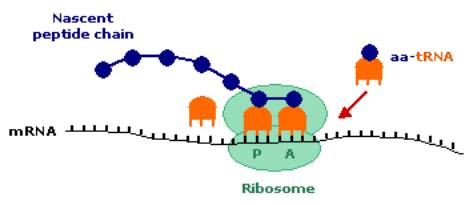
During elongation, the tRNA attached to the growing peptide chain is on the P-site of the small ribosomal subunit.† The incoming aminoacyl-tRNA (aa-tRNA) binds to the A-site of the small ribosomal subunit.† The peptidyl-tRNA on the P-site donates the peptide chain to the aa-tRNA on the A-site through peptide bond formation.† Following this transfer, the A-site tRNA moves with the peptide to the P-site, while the deacylated tRNA on the P-site moves to the E-site (exit site), before detaching from the ribosome.† This leaves the A-site free for the next aa-tRNA to bind.† The elongation procedure is carried out by three important proteins known collectively as elongation factors.
†
|
|
|
Peptide synthesis:† the peptide-tRNA on the P-site of the ribosome is shown transferring its peptide chain to the aa-tRNA on the A-site.† The next aa-tRNA is shown waiting for the A-site to become vacant before binding. |
Elongation Factors, Accurately
Translating mRNA
††††††††††† Elongations factors are largely responsible for achieving accuracy during the translation of the mRNA code into an amino acid sequence.† The correct aa-tRNA must be selected to bind the exposed mRNA codon.† In addition, the mRNA must be moved along the ribosome with precision in order to expose the next codon in the sequence without skipping a nucleotide, which would alter the reading frame and therefore the amino acids selected to build the protein.† Together, the three elongation factors, EF1A (or EF-Tu), EF1B (or EF-Ts) and EF2 (or EF-G), are responsible for all these steps.† Elongation factors are remarkably conserved throughout evolution, EF1A and EF2 being very similar between prokaryotes and eukaryotes, though EF1B is more complex in eukaryotic organisms.
Next:† Elongation Factors in more detail