Cholera Toxin
By Jennifer McDowall
Link to the structural features of cholera toxin
|
|
|
Cholera is an infectious intestinal disease characterised by extreme diarrhoea, vomiting and cramps, often leading to death. Outbreaks of cholera have been recorded as early as during the times of Sanskrit writings, and have continued to be prevalent on the Indian subcontinent for centuries. During the 19th century, cholera spread to Europe and the Americas, causing several devastating epidemics. The progress of the illness in a cholera victim was a frightening spectacle: the quick onset of diarrhoea increased in intensity and was accompanied by retching, extreme thirst, painful abdominal cramps and a change in skin colour to a blue-grey as the blood thickened with the loss of fluid. By tracing an outbreak of cholera in London in 1854 to a particular water pump in Soho, John Snow was able to show that cholera was being transmitted through water. Thirty years later, Robert Koch isolated the culprit: the bacterium Vibrio cholerae.
|
|
|
|
|
|
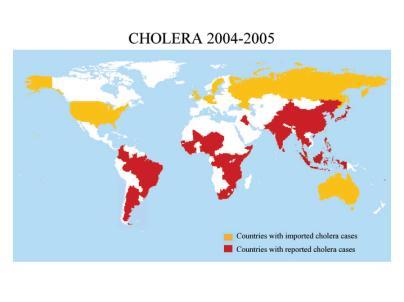
The result of these findings was a widespread clean up of the sewer systems and the containment of stagnant water in most European cities, as well as the development of effective medical rehydration treatments. Yet today, cholera continues to be a serious health problem in Africa, Asia and Latin America, with as many as 200,000-500,000 cases per year, and mortality rates reaching as high as 20-50%. There are currently no effective prophylactics, and treatment by oral rehydration is often foiled by the lack of clean water supplies. Therefore, current research aims include the targeting of cholera toxin itself, especially the inhibition of its binding to receptors in the intestine and the development of anti-secretory agents.
Cholera Toxin, The Main Culprit
|
|
|
Cholera toxin |
|
The debilitating loss of intestinal fluid is caused primarily by the release of cholera toxin, the main virulence factor of the pathogen Vibrio cholerae. Cholera toxin is produced by the CTXf bacteriophage residing within the bacteria. Cholera toxin consists of an A subunit coupled to a B subunit: the A subunit consists of an A1 domain containing the enzymatic active site, and an A2 domain that has an a-helical tail, while the B subunit contains five identical peptides that assemble into a pentameric ring surrounding a central pore. The two assemble by the A2 a-helical tail inserting into the pore formed by the B pentamer. This AB5 structure is closely related to the heat-labile enterotoxin (LT) from enterotoxigenic Escherichia coli, which causes diarrhoea, often in infants. The A and B subunits of cholera toxin are produced in the cytosol by two genes that overlap by one base, and are then assembled into a toxin in the periplasm located between the inner membrane and the outer cell wall of the bacteria. The A subunit in the A/B cholera toxin complex is inactive, and requires the cleavage of the A1 domain from the A2 domain within the infected cell for A1 to become an active enzyme.
Next: The Actions of Cholera Toxin