Unraveling The Molecular Mysteries Of Parkinson's Disease: Key Proteins And Their Roles
focusParkinson’s disease (PD) is a complex neurodegenerative disorder that affects millions worldwide. As our population ages, understanding this condition becomes increasingly crucial. In this post, we’ll delve into the molecular underpinnings of PD, focusing on the key proteins that play pivotal roles in its development and progression.
What is Parkinson’s Disease?
Parkinson’s disease (PD) is a progressive neurodegenerative disorder primarily characterised by motor symptoms such as tremor, rigidity, bradykinesia, and postural instability [1,2]. It typically affects older individuals, with a prevalence of 0.5-1% among those aged 65-69, increasing to 1-3% in those 80 years and older [1,2].
PD progression varies among individuals, but generally follows a pattern of increasing symptom severity over time [3,4]. PD progression begins with a lengthy preclinical phase, characterised by α-synuclein protein misfolding and aggregation at the molecular level, occurring approximately 20 or more years before any noticeable symptoms appear. This is followed by the prodromal phase, spanning 5-20 years before clinical diagnosis, during which individuals experience early non-motor symptoms such as loss of smell, sleep disorders (particularly REM sleep behaviour disorder), constipation, and depression. During this period, α-synuclein continues to accumulate in the brain, and mitochondrial dysfunction begins to develop [5]. These early stages highlight that significant molecular and cellular changes occur long before the characteristic motor symptoms that typically lead to a Parkinson’s disease diagnosis. The disease is often described in stages significantly impacting quality of life over time, with early stages (1-2) involving mild symptoms affecting one or both sides of the body, progressing to middle stages (3) where balance becomes impaired, and advanced stages (4-5) characterised by severe disability and potential confinement to a wheelchair [3,4]. Non-motor symptoms, including cognitive impairment, sleep disorders, and autonomic dysfunction, often emerge and worsen as the disease advances [1,2,5]. While the rate of progression differs for each person, it’s estimated that most individuals advance one stage approximately every two years, with stage 2 typically lasting about five years [3,4].
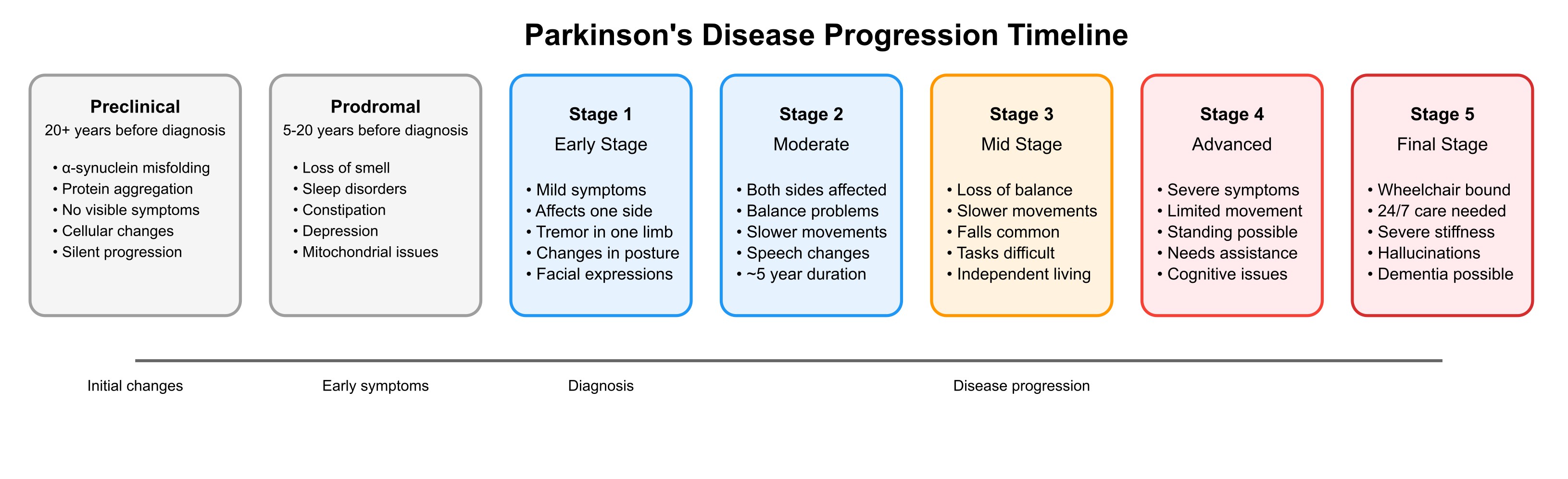 Figure 1. Parkinson’s disease progression stages and associated symptoms (generated by Claude AI).
Figure 1. Parkinson’s disease progression stages and associated symptoms (generated by Claude AI).
The origin of Parkinson’s disease
At its core, PD primarily originates from the loss of dopaminergic neurones in the substantia nigra, a region of the midbrain [6,7]. This loss leads to a deficiency of dopamine, a crucial neurotransmitter for motor control. The degeneration of these neurones is associated with the accumulation of abnormal protein aggregates, particularly α-synuclein, which forms Lewy bodies [6,5].
PD’s development results from a complex interaction of genetic predispositions in 10-15% of PD’s cases (involving genes like SNCA, LRRK2, VPS35, PRKN, PINK1, and DJ1), environmental factors (such as exposure to pesticides), and age-related neurological changes, with the average age of onset being 60 years [6].
Key proteins in Parkinson’s Disease
Several proteins have been shown to play crucial roles in Parkinson’s disease (PD) pathology.
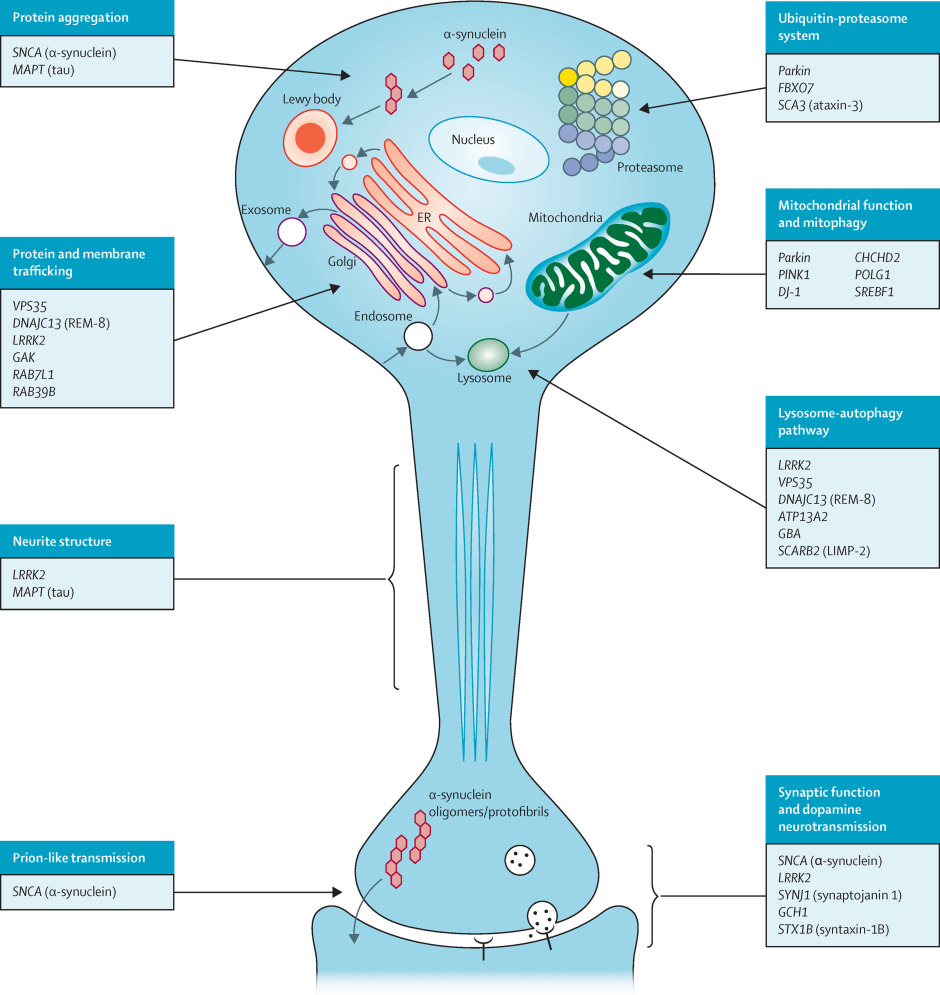 Figure 2. Cellular processes involved in the pathogenesis of Parkinson’s disease [8].
Figure 2. Cellular processes involved in the pathogenesis of Parkinson’s disease [8].
α-synuclein
α-synuclein (α-Syn, IPR002460) is a central protein in Parkinson’s disease (PD) pathology. It forms abnormal aggregates and Lewy bodies in the brain, interacts with vesicles and P-body structures to regulate gene expression through mRNAs, and modulates dopamine release and synaptic function [9]. The accumulation of α-Syn leads to mitochondrial impairment and lysosomal dysfunction [10].
LRRK2
LRRK2 (Leucine-rich repeat kinase 2, UniProt Q5S007) is another crucial protein in PD. Genetic mutations in LRRK2 are associated with both familial and sporadic PD [11]. This protein is involved in various cellular processes, including vesicle trafficking and autophagy. The phosphorylation status of LRRK2 significantly influences its interactions with other proteins, including 14-3-3 proteins, which are found within Lewy bodies in PD patients [8]. The LRRK2 monomer consists of seven distinct domains: ARM (Armadillo repeat, PF23744), ANK (Ankyrin repeat, PF23745), LRR (Leucine-rich repeat, IPR001611), ROC (Ras of complex proteins, IPR020859), COR (C-terminal of ROC, IPR032171), Kinase domain (IPR000719) and WD40 domain (PF23748). The N-terminal domains (ARM, ANK, and LRR) and the C-terminal WD40 domain are primarily involved in protein-protein interactions and regulation of the catalytic core [12,13].
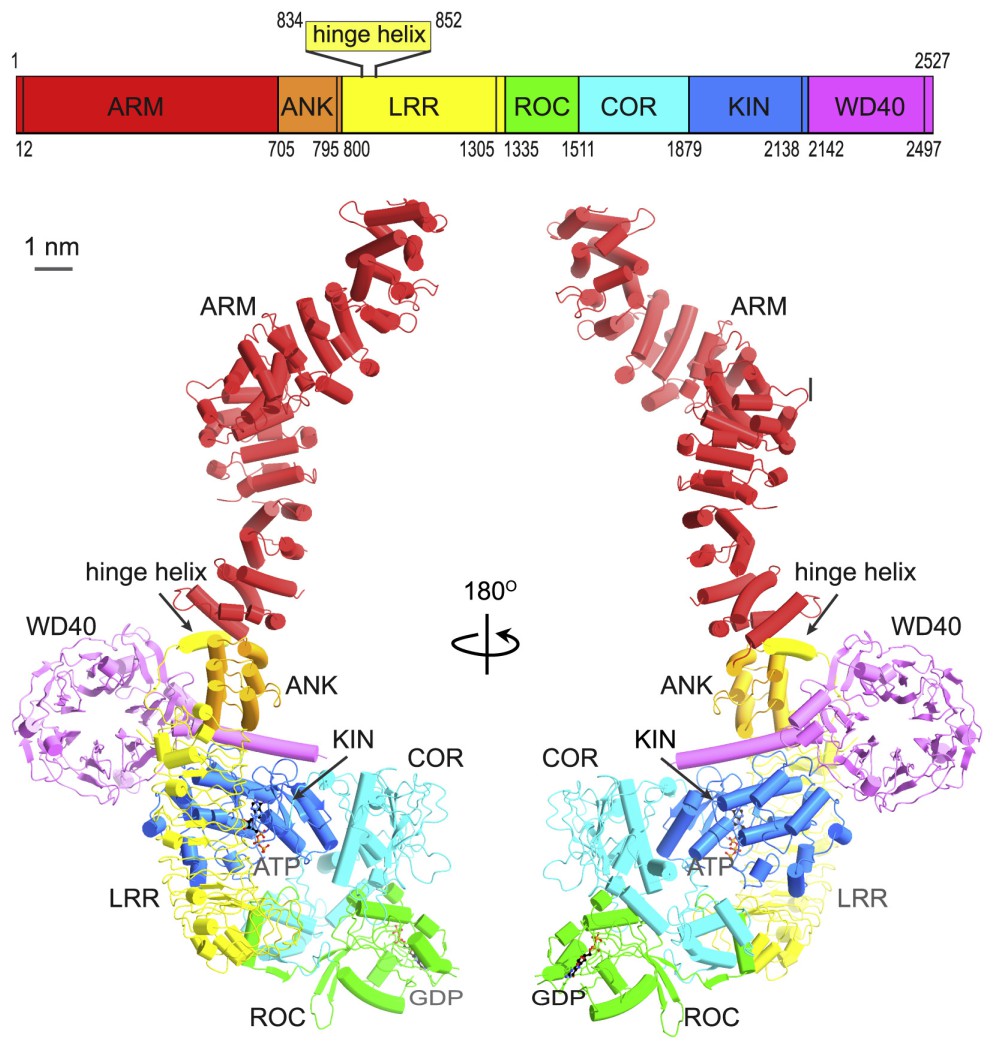 Figure 3. LRRK2 domain composition (adapted from [14]).
Figure 3. LRRK2 domain composition (adapted from [14]).
Parkin
Parkin (IPR019399), an E3 ubiquitin ligase involved in protein degradation, plays a significant role in PD pathogenesis. Mutations in the PRKN gene encoding Parkin are linked to early-onset PD. Working in conjunction with Parkin is PINK1 (PTEN-induced kinase 1, IPR040110), a mitochondrial serine/threonine-protein kinase. Together, these proteins are crucial for mitochondrial quality control [15].
14-3-3 proteins
14-3-3 proteins (IPR000308) play a significant role in PD pathogenesis. These highly conserved proteins are found within Lewy bodies, the hallmark protein aggregates in PD [16,17]. 14-3-3s interact with key PD-associated proteins, including α-synuclein, LRRK2, and Parkin, regulating their activity and localisation [16,17]. They exhibit neuroprotective effects in several PD models, with some isoforms showing stronger protective capabilities [16,18]. However, alterations in 14-3-3 phosphorylation, particularly at serine 232, have been observed in PD brains and may contribute to the neurodegenerative process by reducing 14-3-3’s neuroprotective function [18,19].
DJ-1
DJ-1 (also known as Park7, IPR006287) is another protein implicated in PD, primarily involved in oxidative stress response. Mutations in DJ-1 are associated with early-onset PD, highlighting its importance in neuroprotection [20].
Vps35
Vps35 (Vacuolar protein sorting-associated protein 35, IPR005378), a component of the retromer complex involved in intracellular trafficking, has been linked to late-onset PD through genetic mutations [21].
Rab35
Rab35 (IPR041815), a small GTPase protein, plays crucial roles in intracellular membrane trafficking and has recently been implicated in PD pathogenesis. Rab35 is a member of the Rab family (IPR050227), it has GTPase activity and can bind to GDP and GTP [22]. Studies have shown increased Rab35 expression in PD patients’ serum and substantia nigra, suggesting its potential as a biomarker for the disease [23]. Rab35 interacts with LRRK2 and its phosphorylation by LRRK2 regulates α-synuclein propagation [24]. Overexpression of Rab35 promotes the aggregation and secretion of mutant A53T α-synuclein in dopaminergic cells, exacerbating cell death. Combined assessment of serum α-synuclein and Rab35 levels has shown promise in discriminating PD patients from healthy controls and those with atypical parkinsonian disorders [25].
GBA
Lastly, GBA (Glucocerebrosidase), a lysosomal enzyme, has been found to play a role in PD pathogenesis. GBA is a member of the glycoside hydrolase family 30 (IPR001139) and consists of three distinct domains (I-III) [26]. Domain I forms a three-stranded anti-parallel β-sheet and is responsible for the catalytic activity of the enzyme. Domain II consists of two β-sheets that resemble an immunoglobulin fold. Domain III (residues 76–381 and 416–430) is homologous to a TIM barrel and is a highly conserved domain among glycoside hydrolases [27]. The active site is located in a cavity formed within Domain III, where the substrate glucocerebroside binds near the catalytic residues [26]. Mutations in the GBA1 gene are found in 5-15% of PD cases and increase PD risk. This underscores the importance of lysosomal function in the disease process [6].
These proteins are part of an intricate network of cellular processes, including synaptic function, protein degradation, mitochondrial health, and cellular trafficking. Their dysfunction contributes to the complex pathology of Parkinson’s disease, leading to the progressive loss of dopaminergic neurones and subsequent motor and non-motor symptoms [15,16].
Current therapeutic approaches
Understanding these molecular interactions is crucial for developing targeted therapies. Current therapeutic approaches for PD are evolving from traditional symptomatic treatments toward targeted molecular interventions. A particularly promising area is the development of LRRK2 kinase inhibitors, which aim to modulate the activity of this key protein implicated in both familial and sporadic PD [28]. Another significant focus is on strategies to prevent or reduce α-synuclein aggregation, including antibody-based therapies and small molecules that target its misfolding and accumulation [29]. Emerging approaches also include targeting GBA enzyme activity to improve lysosomal function and developing treatments that enhance mitochondrial function through the PINK1/Parkin pathway [30]. Additionally, researchers are exploring the potential of 14-3-3 proteins as therapeutic targets due to their neuroprotective effects and interactions with multiple PD-associated proteins [17]. These molecular-targeted approaches represent a shift toward potential disease-modifying treatments that could slow or halt disease progression rather than merely managing symptoms.
Conclusion
The journey to unravel the mysteries of Parkinson’s disease is ongoing. As we continue to identify and understand the roles of key proteins involved in PD, we move closer to developing more effective treatments and potentially even preventive strategies. The complex interplay of genetic, environmental, and age-related factors in PD underscores the need for a multifaceted approach to research and treatment.
Disclaimer: This post has been written with the help of Perplexity.ai and its content and sources have been verified and updated when necessary.
References
1.Kouli A, Torsney KM, Kuan W-L. Parkinson’s Disease: Etiology, Neuropathology, and Pathogenesis. Parkinson’s Disease: Pathogenesis and Clinical Aspects [Internet]. Codon Publications; 2018.
2.Jankovic J, Tan EK. Parkinson’s disease: etiopathogenesis and treatment. J Neurol Neurosurg Psychiatry. 2020;91: 795–808.
3.Parkinson’s Community Help. “The Parkinson’s Journey: Understanding Progression” – Webinar Notes Stanford PD Community Blog. 16 Sep 2021 [cited 27 Jan 2025]. Available: https://parkinsonsblog.stanford.edu/2021/09/the-parkinsons-journey-understanding-progression-webinar-notes/
4.Stages of Parkinson’s. In: Massachusetts General Hospital [Internet]. [cited 27 Jan 2025]. Available: https://www.massgeneral.org/neurology/treatments-and-services/parkinsons-disease/stages
5.Zafar S, Yaddanapudi SS. Parkinson Disease. StatPearls [Internet]. StatPearls Publishing; 2023.
6.Parkinson’s disease. Wikimedia Foundation, Inc.; 22 Jul 2001 [cited 27 Jan 2025]. Available: https://en.wikipedia.org/wiki/Parkinson%27s_disease
7.Causes. In: Parkinson’s Foundation [Internet]. [cited 27 Jan 2025]. Available: https://www.parkinson.org/understanding-parkinsons/causes
8.Website. Available: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(14)61393-3/fulltext#fig3
9.The Parkinson’s disease protein alpha-synuclein is a modulator of processing bodies and mRNA stability. Cell. 2022;185: 2035–2056.e33.
10.Burbulla LF, Song P, Mazzulli JR, Zampese E, Wong YC, Jeon S, et al. Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson’s disease. Science. 2017 [cited 27 Jan 2025]. doi:10.1126/science.aam9080
11.Zach S, Felk S, Gillardon F. Signal Transduction Protein Array Analysis Links LRRK2 to Ste20 Kinases and PKC Zeta That Modulate Neuronal Plasticity. PLOS ONE. 2010;5: e13191.
12.Zhang X, Kortholt A. LRRK2 Structure-Based Activation Mechanism and Pathogenesis. Biomolecules. 2023;13: 612.
13.Leschziner AE, Reck‐Peterson SL. Structural Biology of LRRK2 and its Interaction with Microtubules. Movement Disorders. 2021;36: 2494.
14.Structural analysis of the full-length human LRRK2. Cell. 2021;184: 3519–3527.e10.
15.Gómez-Benito M, Granado N, García-Sanz P, Michel A, Dumoulin M, Moratalla R. Modeling Parkinson’s Disease With the Alpha-Synuclein Protein. Front Pharmacol. 2020;11: 524328.
16.Giusto E, Yacoubian TA, Greggio E, Civiero L. Pathways to Parkinson’s disease: a spotlight on 14-3-3 proteins. npj Parkinson’s Disease. 2021;7: 1–14.
17.Abdi G, Jain M, Patil N, Upadhyay B, Vyas N, Dwivedi M, et al. 14-3-3 proteins—a moonlight protein complex with therapeutic potential in neurological disorder: in-depth review with Alzheimer’s disease. Front Mol Biosci. 2024;11: 1286536.
| 18.14-3-3 Phosphorylation as a Biomarker for Parkinson’s Disease. In: The Michael J. Fox Foundation for Parkinson’s Research | Parkinson’s Disease [Internet]. [cited 27 Jan 2025]. Available: https://www.michaeljfox.org/grant/14-3-3-phosphorylation-biomarker-parkinsons-disease |
19.Slone SR, Lavalley N, McFerrin M, Wang B, Yacoubian TA. Increased 14-3-3 phosphorylation observed in Parkinson’s disease reduces neuroprotective potential of 14-3-3 proteins. Neurobiology of disease. 2015;79. doi:10.1016/j.nbd.2015.02.032
20.Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, et al. Mutations in the DJ-1 Gene Associated with Autosomal Recessive Early-Onset Parkinsonism. Science. 2003 [cited 27 Jan 2025]. doi:10.1126/science.1077209
21.Williams ET, Chen X, Moore DJ. VPS35, the Retromer Complex and Parkinson’s Disease. Journal of Parkinson’s Disease. 2017;7: 219.
22.Rab35 Regulates an Endocytic Recycling Pathway Essential for the Terminal Steps of Cytokinesis. Current Biology. 2006;16: 1719–1725.
23.Chiu CC, Yeh TH, Lai SC, Weng YH, Huang YC, Cheng YC, et al. Increased Rab35 expression is a potential biomarker and implicated in the pathogenesis of Parkinson’s disease. Oncotarget. 2016;7. doi:10.18632/oncotarget.11090
24.Bae E-J, Kim D-K, Kim C, Mante M, Adame A, Rockenstein E, et al. LRRK2 kinase regulates α-synuclein propagation via RAB35 phosphorylation. Nature Communications. 2018;9: 1–16.
25.Wang H-L, Lu C-S, Yeh T-H, Shen Y-M, Weng Y-H, Huang Y-Z, et al. Combined Assessment of Serum Alpha-Synuclein and Rab35 is a Better Biomarker for Parkinson’s Disease. Journal of Clinical Neurology. 2019;15: 488–495.
26.Website. doi:10.4061/2011/973231
27.Rigden DJ, Jedrzejas MJ, de Mello LV. Identification and analysis of catalytic TIM barrel domains in seven further glycoside hydrolase families. FEBS Letters. 2003;544: 103–111.
| 28.Role of LRRK2 in Alpha-Synuclein-Induced Neurodegeneration. In: The Michael J. Fox Foundation for Parkinson’s Research | Parkinson’s Disease [Internet]. [cited 27 Jan 2025]. Available: https://www.michaeljfox.org/grant/role-lrrk2-alpha-synuclein-induced-neurodegeneration |
29.Lee H-J, Bae E-J, Lee S-J. Extracellular α-synuclein—a novel and crucial factor in Lewy body diseases. Nature Reviews Neurology. 2014;10: 92–98.
30.Lee H, Elkamhawy A, Rakhalskaya P, Lu Q, Nada H, Quan G, et al. Small Molecules in Parkinson’s Disease Therapy: From Dopamine Pathways to New Emerging Targets. Pharmaceuticals (Basel). 2024;17: 1688–1688.