SRC, proto-oncogene
tyrosine-protein kinase
Src
regulation: conformational opening and activation
Reprinted from Biochim Biophys Acta 1602(2), M. Frame, Src in Cancer, 114-130, 2002, with permission from Elsevier, PMID: 12020799
How Src works
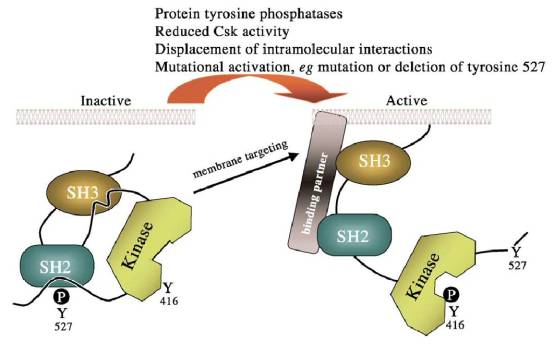
The Src protein has three major domains, SH2 (for Src homology 2), SH3, and the kinase catalytic domain (or SH1), as shown above. SH2 and SH3 both play a part in protein-protein interactions, while the kinase catalytic domain contains the kinase active site. Src can be switched from an inactive to an active state through control of its phosphorylation state, or through protein interactions. There are two major phosphorylation sites on Src: one is at Tyr416 (or Y416), the other at Tyr527, as marked in the drawing above for chicken Src. Tyr416 can be auto-phosphorylated, which activates Src by displacing the P-Tyr416 from the binding pocket, allowing the substrate to gain access. A more critical site is Tyr527, which can be phosphorylated and dephosphorylated by various proteins, such as CSK kinase (phosphorylates), or SHP-1 phosphorylase (dephosphorylates). Phosphorylation of Tyr527 inactivates Src through the interaction of P-Tyr527 with the SH2 domain, which effectively folds Src up into a closed, inaccessible bundle. Dephosphorylation of Tyr527 releases this bond, opening up the molecule to an active state. Protein interactions also act to regulate Src by either directly activating Src, or by moving Src to sites of action. Both platelet-derived growth factor and focal adhesion kinase are able to bind to the SH2 domain, causing Src to open up into the active form.
Many of the substrates that Src can phosphorylate with its kinase domain form part of signalling cascades. These include Fak and Cas, which are important for integrin signalling, as well as Shc and Stat3, which are involved in growth regulation. Signalling systems often involve a cascade mechanism of sequential phosphorylation and dephosphorylation of proteins in the cascade, as occurs here.
Src family
members
Considering the vital role Src
seems to play in cell signalling, scientists were surprised to find that mice
deficient in Src could survive. The
reason for this result is that Src is one of nine members in a closely-related
family that can often compensate for one another. Other Src family kinase members are Fyn and Yes, which like Src
are widely expressed, and Blk, Fgr, Hck, Lck, Lyn, and Yrk, which are expressed
in specific tissues. All these proteins
have structurally similar SH2, SH3 and kinase domains and are capable of acting
as signalling molecules in a similar manner to Src.
When Src goes wrong
Under normal circumstances, Src is predominantly inactive in cells, being switched on only at specific times. However, if the fine balance between phosphorylation and dephosphorylation is disrupted, changes can occur in Src activity with drastic results. Several cancers, including colon and breast cancer, have been associated with an increase in Src activity. In fact, Src was first isolated as an oncogene, v-Src, from the transforming virus, Rous Sarcoma Virus. v-Src was found to lack the region of the cellular protein (c-Src) that contains Tyr527, making it continually active.
In a similar fashion, c-Src can become abnormally active, either through mutations in c-Src itself, or through mutations in proteins that regulate c-Src. In late stage colon cancers, mutations have been reported in the src gene that cause the loss of the region containing Tyr527, leading to Src over-activity. Proteins that regulate Src have also been found at abnormal levels in cancer cells, including both those that activate and those that inactivate Src. Proteins such as PTPalpha, SHP-1 and PTP1B that activate Src by dephosphorylating Tyr527 have been detected at elevated levels in various cancer cells, including epidermal and breast carcinoma cells. Conversely, proteins such as Csk and Chk that inactivate Src by phosphorylation of Tyr527 have been detected at reduced levels in certain cancer cells. As such, proteins like Csk and Chk are considered to have a tumour-suppressing ability.
With its
importance in cell regulation, and its implication in cancer, Src, as well as
other protein kinases, has become an important drug target in the battle
against cancer.