Calmodulin
Courtesy of Danton H. O’Day. Cellular Signalling 15, D. O’Day, CaMBOT: profiling and characterizing calmodulin-binding proteins, 347-354 (2003), with permission from Elsevier, PMID: 12618209
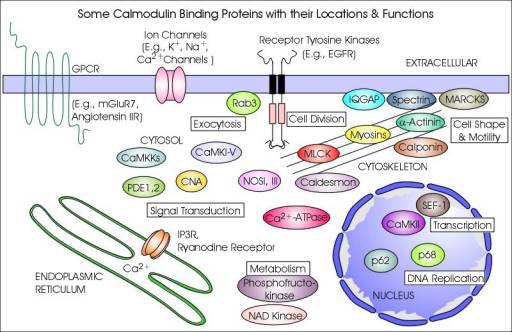
CaM-binding proteins
CaM-binding proteins (CaMBP) are a diverse group of targets, whose interactions with CaM can be subdivided into calcium-dependent and calcium-independent modes of binding and regulation. Ca2+-CaM can bind to target proteins to alter their function, acting as part of a calcium signal transduction pathway. Calcium-free apocalmodulin (ApoCaM) can bind to other target proteins, eliciting different cellular responses.
There is no general model of target recognition and activation by CaM, instead several mechanisms of interaction have been described for different targets. CaM is thought to activate myosin light chain kinase (MLCK) and CaM kinase II (CaMKII) by displacement of their auto-inhibitory domains. With anthrax adenylate cyclase exotoxin activation, CaM was found to insert itself between two domains of the exotoxin. Other mechanisms of activation include binding of the CaM C-terminal alone, binding of CaM in an extended conformation, and dimer formation with its target. This mechanistic diversity means that there is no conserved amino acid sequence for CaM binding, however several different binding motifs have been recognised based on the positioning of conserved hydrophobic residues. CaM binding proteins can have one or more CaM-binding domains.
Ca2+-CaM-binding proteins
Ca2+-CaM can target several different types of proteins, as shown in the table. Ca2+-CaM binding proteins include kinases, phosphatases, second messenger signalling proteins, cytoskeletal and muscle proteins. These proteins can be either Ca2+-activated or Ca2+-inhibited. Calcium-dependent responses include neurotransmitter release, muscle contraction, metabolism and the inflammatory response. Some of the responses are outlined below:
CaM kinase cascade
Ca2+-CaM can activate CaM kinase kinase (CaMKK), which in turn activates CaM kinase I (CaMKI) and CaMKIV, enabling them to phosphorylate various protein targets. CaMKIV can activate several transcription factors such as CREB, SRF, and CBP in order to selectively activate the transcription of target genes. CaMKK can also phosphorylate protein kinase B, thereby inhibiting apoptosis.
Calcium channel control
CaM is able to exert two opposing effects on P/Q-type calcium channels, first promoting then inhibiting channel opening. Both of these responses require Ca2+-CaM to bind to the C-terminal tail of the channel. The different effects arise from which CaM domain binds the calcium: Ca2+-binding to the C-terminal CaM domain promotes channel opening, while Ca2+-binding to the N-terminal CaM domain promotes channel closure. The modulation of Ca2+ influx via the control of calcium channels can have important consequences. For instance, Ca2+ is essential for cardiac excitation-contraction coupling and the propulsion of blood: Ca2+ activates myofilaments during cardiac systole causing contraction; the release of Ca2+ from the myofilaments during cardiac diastole then causes relaxation.
ApoCaM-binding proteins
ApoCaM is structurally different from Ca2+-CaM and utilizes unique binding motifs, which show greater homology than Ca2+-CaM binding motifs. ApoCaM targets several proteins, as shown in the table. ApoCaM binding proteins include neuroproteins, receptors, second messenger signalling proteins, cytoskeletal and muscle proteins. Calcium-independent responses include neurotransmitter production, nerve growth, synaptic development, intracellular movement and smooth muscle relaxation. Some of the responses are outlined below:
ApoCaM storage by neuromodulin
Neuromodulin is the only known protein in the membrane to have a higher affinity for ApoCaM than for Ca2+-CaM. Calcium-dependent phosphorylation of neuromodulin prevents ApoCaM binding. Neuromodulin binds and concentrates ApoCaM at specific sites and releases it in response to Ca2+ or phosphorylation, thereby controlling the concentration of ApoCaM in neurons.
Intracellular movement
Brush border myosins (myosin Ia) contain a motor domain that hydrolyses ATP as the driving force for its translocation along actin filaments. These proteins are structurally different from the more common myosins. Myosin Ia preferentially binds ApoCaM to Ca2+-CaM. ApoCaM activates ATPase activity in the absence of actin, thereby inhibiting motiliy.
Next: What InterPro tells us
Previous: Calmodulin, a signal transducer