SV40
|
|
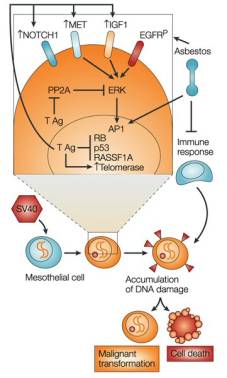
SV40 and the pathogenesis of mesothelioma Diagram shows an in vitro transformation model, where asbestos and SV40 large-T Ag are acting as co-carcinogens. T-Ag is shown to block p53, RB and RASSF1A, and upregulate Notch1 and MET oncogenes. Reprinted by permission from Nature Reviews
Cancer 2:957-964, A. Gazdar, J. S. Butel and M. Carbone "SV40 and tumours: myth, association or causality?" copyright (2002) Macmillan Magazines Ltd |
SV40 and cancer
Several viruses have been implicated in the pathogenesis of different cancers, including papillomavirus, Epstein-Barr virus, hepatitis B virus and HIV-1. The study of tumour-promoting viruses has greatly increased our understanding of cancer pathology. SV40 has been found in various tumour types, including brain tumours, bone tumours, mesotheliomas and non-Hodgkin’s lymphomas, suggesting that SV40 maybe a transforming virus under certain circumstances. All these tumour-types can be induced with SV40 in laboratory animals.
The SV40 small-t Ag and large-T Ag are considered to be oncoproteins under certain conditions, and together they can transform cells in culture. The transformation of cultured cells requires both the stimulation of cell division and the blocking cell apoptosis; the large-T Ag can bring both of these functions about.
The large-T Ag is a multifunctional protein concerned with a wide range of cellular processes, including transcriptional activation and repression, blocking of differentiation, stimulation of the cell cycle, repression of apoptosis and cell transformation. The large-T Ag has three domains: a DnaJ domain (acts as a molecular chaperone), a RB-binding domain and a p53-binding domain, while the small-t Ag has the DnaJ domain and a serine/threonine phosphatase PP2A-binding domain. The large-T Ag gains control of the cell through its interactions with cellular proteins such as p53, RB (retinoblastoma), p107, p130, CBP/p300, and RASSF1A. The large-T Ag is able to disrupt both the RB and the p53 tumour suppressor pathways by binding and inactivating the cell cycle control proteins RB and p53, which stimulates the host cell to enter S phase and undergo DNA synthesis. RB acts to arrest cells in the G1 phase of cell division by repressing the transcription of genes required for entry into S phase, while p53 controls an apoptosis pathway; by inactivating both these proteins, the large-T Ag is able to stimulate cell division and block apoptosis.
The small-t Ag acts to transform cell by binding to PP2A, an abundant family of serine-threonine phosphatases. The loss of PP2A activity is thought to cause defects in the biogenesis and properties of tight junctions, leading to the disorganisation of the actin cytoskeleton. PP2A loss also causes the deregulation of Rho GTPases, F-actin and intercellular adhesion. Defects in the actin cytoskeleton and the disruption of tight junctions have been linked to tumour invasiveness.
Next: What InterPro tells us
Previous: Architecture of a virus