Serpins:
a1-Antitrypsin
By Jennifer McDowall
To view a1-antitrypsin structure
Proteases play an essential role in the life of an organism, acting to cleave peptide bonds in various proteins through hydrolysis. The many different classes of proteases underscore the crucial role that they play, each with related but distinctive functions. Some serve to convert inactive forms of a protein into their active counterparts, while others act as a means of defence against intruding pathogens. Many proteases form part of proteolytic cascades, where the product from one reaction acts as the substrate for the next, thereby amplifying an initial signal into a large response. Proteases are classified according to the nature of their active site, such as serine proteases, threonine proteases, aspartic proteases, cysteine proteases, and metalloproteases.
However, unchecked these proteases could cause catastrophic effects, literally digesting tissues with which they come into contact. Nature has solved this problem by producing an array of protease inhibitors that evolved in parallel with the proteases they control. One such family of inhibitors are the serpins (serine protease inhibitors), which control the activity of serine proteases, as well as of other protease molecules. Serpins have developed a sophisticated means of blocking protease activity, which is unique among the different types of protease inhibitors.
a1-antitrypsin, the archetypical serpin
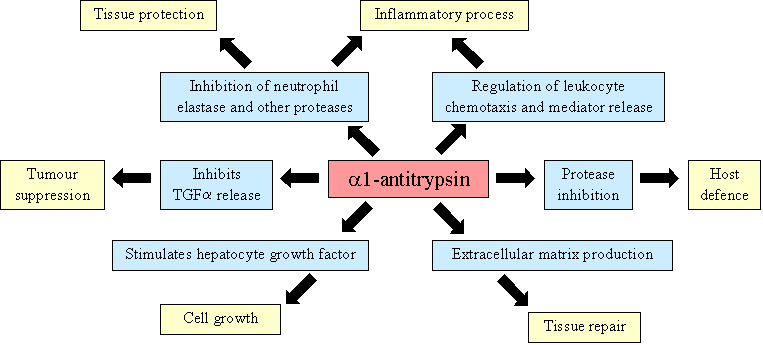
a1-antitrypsin (also known as a1-antiproteinase) is the main plasma protease inhibitor, accounting for as much as 70% of the serpins circulating in plasma. During inflammation a1-antitrypsin levels can rise even further, with the circulating enzyme reaching 3-times its normal level. Synthesized in the liver and macrophages, a1-antitrypsin can inhibit a broad range of proteases, including trypsin, chymotrypsin, thrombin, kallikrein and plasmin. However, its primary target is neutrophil elastase (EC 3.4.21.37), acting to protect the lower respiratory tract from proteolytic destruction by this powerful protease. a1-antitrypsin is thought to have other functions as well. In addition to inhibiting the proteolytic activity of leukocyte elastase, it is thought to directly affect leukocyte chemotaxis and mediator release, and may contribute to the host defence against invading microorganisms. a1-antitrypsin has also been implicated in cell growth and extracellular matrix production, through which it could have a role in tissue repair.
Next: A
balancing act