Serpins: a1-antitrypsin
|
|
|
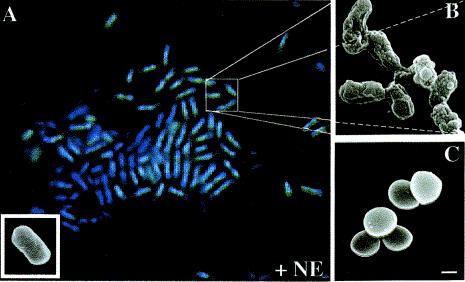
(A) Gram negative E. coli showing damaged membranes (B) after
incubation with neutrophil elastase.
(C) Gram positive S. aureus are unaffected by elastase. Reprinted from Microbes and Infection 4, A.
Belaaouaj, Neutrophil elastase-mediated killing of bacteria: lessons from
targeted mutagenesis, 1259-1264, 2002.
PMID:
12467768. |
A balancing act
The fine balance between a protease and its inhibitor is a major determinant in maintaining tissue integrity. This is exemplified by the interplay between a1-antitrypsin and neutrophil elastase. Neutrophils play a critical role in host defence against invading pathogens. Neutrophils are produced in the bone marrow and are fully mature when released into circulation to take up their role as the first line of cellular defence. Pro-inflammatory mediators and chemotactic attractants activate neutrophils and draw them to the site of infection, where they act to engulf bacteria by phagocytosis, assaulting them with an arsenal of anti-bacterial compounds that use both oxidative and non-oxidative methods of attack. The powerful serine protease, neutrophil elastase, is one of those anti-bacterial compounds that are clearly involved in destroying bacteria. Neutrophil elastase is released into the phagolysome surrounding the microorganism, which it proceeds to destroy. Neutrophil elastase is able to attack the outer membrane protein, OmpA, in gram-negative bacteria (as shown above), helping to directly kill the pathogen by degrading its membrane, as well as enabling other anti-bacterial compounds to gain access to the pathogen. In addition, neutrophil elastase may help process other anti-bacterial compounds, converting them from inactive pro-peptides into their active states, such as for cathelicidin.
Yet neutrophil elastase can also cause problems for its host. It is one of the most destructive enzymes in the body, with the capability of degrading extracellular matrix proteins (including collagens, proteoglycan, fibronectin, platelet receptors, complement receptor, thrombomodulin, lung surfactant and cadherins) and key plasma proteins (including coagulation and complement factors, immunoglobulin, several proteases and protease inhibitors). Under physiological conditions, endogenous protease inhibitors, such as a1-antitrypsin, tightly regulate the activity of neutrophil elastase. However, at inflammatory sites, neutrophil elastase is able to evade regulation, and once unregulated it can induce the release of pro-inflammatory cytokines, such as interleukin-6 and interleukin-8, leading to acute lung injury. It can even impair host defence against infection by degrading phagocyte surface receptors and opsonins. Its negative role is illustrated by its involvement in the tissue destruction and inflammation that characterise numerous diseases, including hereditary emphysema, chronic obstructive pulmonary disease, cystic fibrosis, adult respiratory distress syndrome, ischemic-reperfusion injury and rheumatoid arthritis. Therefore, a1-antitrypsin plays a delicate balancing act, allowing enough neutrophil elastase to be present for host defence, yet at the same time providing an anti-neutrophil elastase screen to prevent excessive enzyme from injuring host tissue.
Next: The suicide pact
Previous: a1-Antitrypsin, the archetypical serpin