Snake venom:
Bungarotoxins
The neuromuscular junction
|
|
|
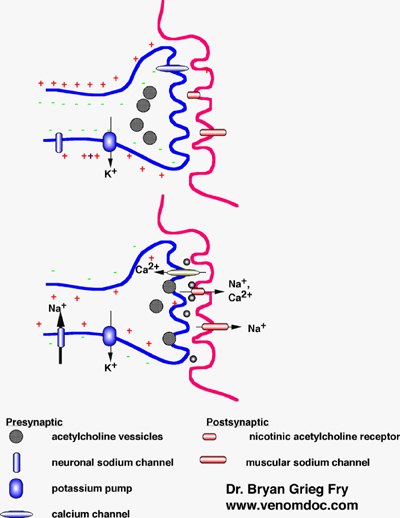
Basic structure of the
neuromuscular junction showing the major channels and structures involved in
nerve transmission. At rest (top),
the cytoplasm of the nerve has a net charge relative to the outside
environment. When discharged
(bottom), the nerve slightly over-shoots, resulting in a slight net positive
charge. Diagram courtesy of B. G. Fry, Australian Venom Research Unit, Melbourne, Australia |
The nervous system sends signals by an electrical impulse that travels along the length of the nerve until it reaches a junction with another nerve cell (synaptic cleft), or a muscle cell (neuromuscular junction). When the impulse reaches a junction, the influx of ions stimulates the release of vesicles containing neurotransmitters, such as acetylcholine, which diffuse across the gap and bind to receptors on the adjoining nerve or muscle cell, thereby continuing the response.
There are two types of acetylcholine receptors (AchR): muscarinic-type, which are primarily neuronal, and nicotinic-type, which are either neuronal or muscle-type. The venom from the many-banded krait (Bungarus multicinctus) contains toxins that can bind to each type of receptor: the a-bungarotoxins act primarily on nicotinic AchRs at the neuromuscular junction, the k-bungarotoxins act primarily on nicotinic AchRs in neuronal tissue, and there are also muscarinic AchR-binding toxins. These toxins show almost irreversible binding to the receptors, competitively inhibiting acetylcholine binding and, consequently, inhibiting the acetylcholine-induced electrical response. Both a- and k-bungarotoxins are three-finger toxins, their characteristic ‘3-finger’ structure being determined by disulphide bonds. This 3-finger fold allows for variation in structure, which can alter the function and selectivity of molecular targets. Evolutionary divergence has given rise to over 100 other post-synaptic a-neurotoxins found in Elapidae and Hydrophiidae, which may be related to the 3-finger proteins of vertebrates that play a significant role in cell-cell adhesion.
Other bungarotoxins
b-Bungarotoxin is much more lethal than either a- or k-bungarotoxin. b-Bungarotoxin is a pre-synaptic toxin that acts on the (pre-synaptic) motor nerve terminals to block the release of acetylcholine. The action of b-bungarotoxin is complex. It has phospholipase A2 activity (EC 3.1.1.4), which functions to hydrolyse phosphatidylcholine, in this case the phospholipids in the nerve membrane. Yet b-bungarotoxin displays both phospholipase-dependent and –independent activities. b-Bungarotoxin is thought to bind to and block Shaker-type potassium channels; the subsequent block of transmitter release is probably due to phospholipase A2-mediated destruction of the nerve terminal. Animals die as a consequence of respiratory failure.
Acetylcolinesterase (AchE) (EC 3.1.1.7) plays a key role in cholinergic nerve transmission, acting to breakdown acetylcholine to choline and acetate, which is important in controlling a receptor’s response. Snake venom makes use of AChE to breakdown any neurotransmitter that might compete with a- or k-bungarotoxin for binding to AchRs. Venom AChE contains an additional exon over endogenous AChE, which generates a soluble form of the enzyme that is suitable for its venomous use.
Venom is an abundant source of nerve growth factor (NGF), which induces neurite outgrowth. Venom NGF is often less potent than mammalian NGF, a family of neurotrophic factors that regulate the survival and differentiation of neurons. Venom NGF acts as a low-potency agonist of TrkA-receptors, thereby competing for binding with endogenous NGF, affecting the survival of cholinergic neurons.
Value of snake toxins in science and medicine
In spite of the harmful effects of snake venom, it has an important place in scientific discovery and medicine. In science, venom toxins provide highly specific research tools. The discovery of a-bungarotoxin led directly to the characterisation of acetylcholine receptors, and is still used for receptor studies. Other toxins have proved useful as ion channel probes, and in the study of inflammation: phospholipase A2 (PLA2) peptides are involved in many inflammatory disorders such as asthma, allergic rhinitis, acute pancreatitis and autoimmune diseases; venom PLA2 offers an opportunity to investigate the mechanism of PLA2 action.
Venom toxins as a natural resource are of medical importance: the myriad of proteins found in venoms has an important place in the treatment of thrombosis, arthritis, cancer and many other diseases. The usefulness of venom toxins lies in their ability to evade regular control mechanisms through their independence from co-factors and their non-recognition by inhibitors. Venom toxins have developed highly specific molecular targets, which make them valuable for drug usage in terms of limiting potential side effects. The considerable divergence of snake toxins has made them a valuable resource for potential lead compounds.
Next: What InterPro tells us
Previous: Venom: an arsenal of toxins