Catalase
How Catalase Works
††††††††††† Most catalases exist as tetramers of 60 or 75 kDa, each subunit containing an active site haem group buried deep within the structure, but which is accessible from the surface through hydrophobic channels.† The very rigid, stable structure of catalases is resistant to unfolding, which makes them uniquely stable enzymes that are more resistant to pH, thermal denaturation and proteolysis than most other enzymes.† Their stability and resistance to proteolysis is an evolutionary advantage, especially since they are produced during the stationary phase of cell growth when levels of proteases are high and there is a rapid rate of protein turnover.
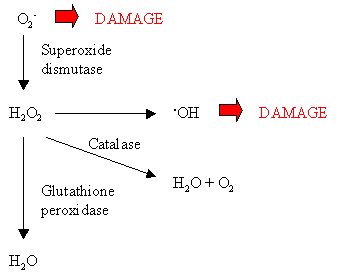
Haem-containing catalases break down hydrogen peroxide by a two-stage mechanism in which hydrogen peroxide alternately oxidises and reduces the haem iron at the active site.† In the first step, one hydrogen peroxide molecule oxidises the haem to an oxyferryl species.† In the second step, a second hydrogen peroxide molecule is used as a reductant to regenerate the enzyme, producing water and oxygen.† Some catalases contain NADPH as a cofactor, which functions to prevent the formation of an inactive compound.† Catalases may have another role: the generation of ROS, possibly hydroperoxides, upon UVB irradiation.† In this way, UVB light can be detoxified through the generation of hydrogen peroxide, which can then be degraded by the catalase.† NADPH may play a role in providing the electrons needed to reduce molecular oxygen in the production of ROS.
Much of the hydrogen peroxide that is produced during oxidative cellular metabolism comes from the breakdown of one of the most damaging ROS, namely the superoxide anion radical (O2-).† Superoxide is broken down by superoxide dismutases into hydrogen peroxide and oxygen.† Superoxide is so damaging to cells that mutations in the superoxide dismutase enzyme can lead to ALS, which is characterised by the loss of motoneurons in the spinal cord and brain stem, possibly involving the activation of caspase-12 and the apoptosis cascade via oxidative stress.†
Regulation of Antioxidant Enzymes
††††††††††† Antioxidant enzymes, including catalase, form the first line of defence against free radicals, therefore their regulation depends mainly upon the oxidant status of the cell.† However, there are other factors involved in their regulation, including the enzyme-modulating action of various hormones such as growth hormone, prolactin and melatonin.† Melatonin is a derivative of the amino acid tryptophan that acts as a neurohormone in mammals, but is also synthesized by many other species, including plants, algae and bacteria.† Melatonin has been shown to markedly protect both membrane lipids and nuclear DNA from oxidative damage.† Melatonin can directly neutralise several ROS, including hydrogen peroxide.† It can also stimulate various antioxidant enzymes, including catalase, either by increasing their activity or by stimulating gene expression for these enzymes.† The decrease in melatonin levels observed with age correlates with an increase in neurogenerative disorders such as Parkinsonís disease, Alzheimerís disease, Huntingtonís disease and stroke, all of which may involve oxidative stress.† In general, the production of ROS increases with aging and is associated with DNA damage to the tissues.
††††††††††† By contrast, growth hormone, and possibly prolactin, was found to decrease catalase and other antioxidant enzymes in various tissues in mice, suggesting that this hormone acts as a suppressor of key antioxidant components.
Next:† What InterPro Tells Us
Previous:† Catalases, Protective Enzymes
†††††††††††