Protein of the Month |
November 2006
MORE ON THIS MONTH’S PROTEIN
|
|
OTHER PROTEINS OF INTEREST |
|
|
|
|
Fibrinogen
By Jennifer McDowall
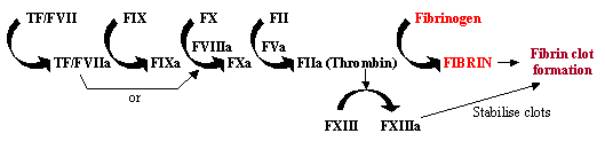
Blood clotting, or coagulation, is a rapid response to tissue damage, where the exposure of tissue factor containing cells to the bloodstream through damage to vessel walls initiates a rapid cascade system. This enzyme cascade uses a series of enzymes to work in rapid succession to amplify a small response into a large one at the site of damage (see Figure 1). Once activated, tissue factor (TF) binds and activates factor VII (FVII), initiating the cascade. TF/FVII is activated through auto-cleavage to TF/FVIIa, which along with cofactor FVIIIa converts FIX to FIXa; FXa can then convert FX to FXa, which along with cofactor FVa converts FII (prothrombin) to FIIa (thrombin); finally FIIa converts fibrinogen to fibrin, leading to fibrin deposition and the activation of platelets to form blood clots. The activation of FXIII to FXIIIa can stabilise these blood clots by cross-linking them.
|
|
|
Figure 1. Blood Coagulation Cascade |
Fibrinogen,
Finishing the Coagulation Cascade
Fibrinogen was first isolated from horse plasma by Hammarsten in 1876, although an inactive precursor to fibrin was proposed to exist as early as 1859 by Deni de Commercy. Fibrinogen can undergo a remarkable transformation from soluble monomers (fibrogen) to an insoluble polymer gel (polymerised fibrin).
Fibrinogen is a plasma glycoprotein synthesised in the liver that is essential for haemostasis (stopping blood loss from damaged tissues), wound healing, fibrinolysis, inflammation, angiogenesis, cellular and matrix interactions, and neoplasia. These processes involve the conversion of fibrinogen to fibrin, and often the interaction of fibrin(ogen) to various proteins and cells. People usually carry about 2.5g fibrinogen/L of blood, however, concentrations of fibrogen can increase by as much as 200-400% during times of physiological stress (primarily due to the actions of macrophage-derived interleukin-6).
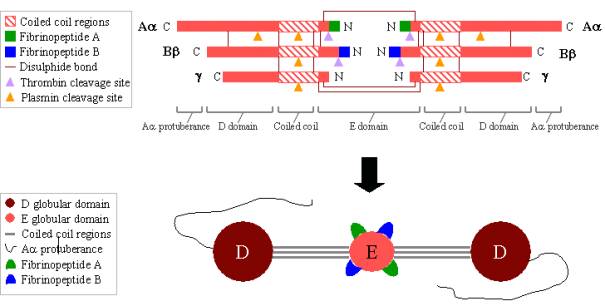
Fibrinogen is a large, complex glycoprotein composed of three pairs of polypeptides: two Aa, two Bb, and two g. These polypeptides are linked together by 29 disulphide bonds, some of which are depicted in Figure 2 below. The polypeptides are oriented so all six N-terminal ends meet to form the central E domain. Two regions of coiled coil alpha helices stretch out on either side of the E domain, each consisting of one Aa, one Bb and one g polypeptide. Each coiled coil region ends in a globular D domain consisting of the C-terminal ends of Bb and g, as well as part of Aa. The C-terminal end of Aa then protrudes from each D domain as a long strand; these Aa protuberances can interact with each other and with the E domain during fibrin clot cross-linking. Both the E and D domains contain important binding sites for the conversion of fibrinogen to fibrin, for fibrin assembly and cross-linking, and for platelet aggregation. Bound calcium ions are important to help maintain the structure of fibrinogen.
The N-terminal ends of both the Aa and Bb polypeptides are cleaved by thrombin in order to turn soluble fibrinogen into gel-forming fibrin. Once cleaved from fibrinogen, the N-terminal ends are known as fibrinopeptide A (from Aa polypeptide) and fibrinopeptide B (from Bb polypeptide).
|
|
|
Figure 2. Fibrinogen: TOP – polypeptide organisation of fibrinogen. BOTTOM - domain organisation of fibrinogen. |
Blood
Clot Formation
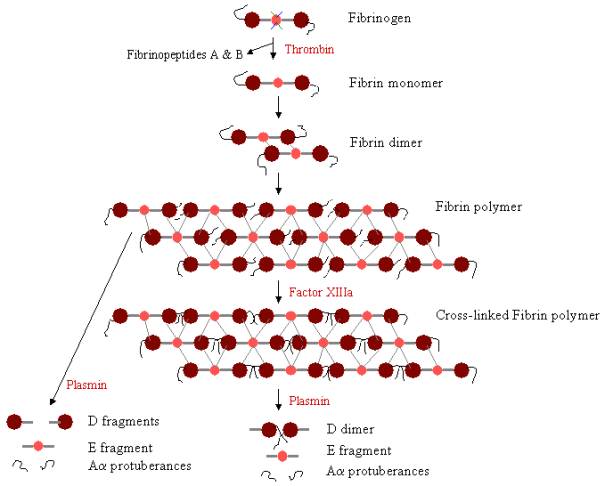
In order to form a blood clot, fibrinogen must first be cleaved by thrombin to remove the fibrinopeptides. The removal of fibrinopeptides A and B from the N-terminal ends of Aa and Bb exposes ‘knobs’ on the E domain, which can interact with the ‘holes’ always present on the D domains. Fibrin molecules can link together through the interaction of the E domain on one fibrin molecule to the D domains on four other fibrin molecules, thereby polymerising to form staggered oligomers that build up into protofibrils. As the fibrin oligomers aggregate, these protofibrils continue to lengthen to make long fibres that can wind around one another to make multi-stranded, thick bundles, and which can branch into a 3-dimentional network of entangled fibres, the fibrin clot. The fibrin clot is then stabilised by Factor XIIIa, a transglutaminase, where the zymogen form of Factor XIII is converted to active Factor XIIIa through the action of thrombin (see Figure 1). Factor XIIIa cross-links glutamine residues on one fibrin molecule to the lysine residues on another fibrin molecule by forming strong isopeptide bonds. This cross-linking occurs between the C-terminal ends (Aa protuberances) of the Aa polypeptides, as well as (more slowly) at other sites, such as between the C-terminal ends of g chains. These cross-links help strengthen the fibrin clot, making it more resistant to physical and chemical damage.
|
|
|
Figure 3. Fibrin Polymerisation and lysis: Pathway of fibrin polymerisation and breakdown. The knobs on the E domain bind to the holes on up to four D domains (grey lines), forming a long, fibrous latticework. The clot is then stabilised through cross-linking. The clot can be degraded, yielding different degradation products if it has been cross-linked. |
Next: Wound Healing