Fibrinogen
Wound
Healing
|
|
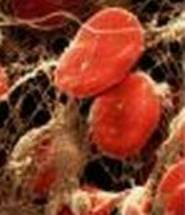
Figure 4. Blood Clot: Fibrin polymers appear as strands surrounding red blood cells. |
The mechanical properties of a blood clot are essential for proper haemostasis and wound healing. A blot clot needs to be strong in order to withstand physical and chemical abrasion, but it also needs to be elastic enough not to crack or split with body movement. In addition, it must also be digestible by lytic enzymes; otherwise, thrombosis could occur as the clot breaks down, which is one of the most common causes of heart attacks and stroke. If thrombosis occurs in an artery, the tissues supplied by that artery would become necrotic, which could lead to myocardial infarction (heart attack) if the thrombosis is in a coronary artery. If the thrombosis occurs in a vein, the tissues draining into that vein would become swollen and inflamed, leading to pulmonary embolism if the clot reaches the heart. By contrast, if the clot is insufficient in size or rigidity, then haemorrhaging can occur.
The characteristics of a blood clot depend upon several factors, especially the structure of the fibrin polymer, including the thickness of the fibres and number of branch points, as well as how permeable it is, which in turn affects its accessibility to incoming repair cells. Its elasticity is due to the ability of long fibrin fibres to bend. In addition, the cross-linking of fibrin polymers renders a clot much stiffer and resistant to being broken down, but this stiffness must be finely controlled.
There are different splice variant of fibrinogen, which can result in low- and high-molecular weight variants, each with their own clotting rate and fibrin polymer characteristics. These naturally occurring variants can modify the ability of endothelial cells to develop new blood cells (angiogenesis) within the fibrin matrix. Therefore, the level of the different variants is an important factor in angiogenesis.
Platelet aggregation
The binding of fibrinogen to platelets is an important part of wound healing by aggregating to form a haemostatic plug and by stimulating the activation of blood coagulation factors. Platelets are one of the main components of blood and play an important role in preventing blood loss through cell adhesion and aggregation to help form a blood clot. They are also involved in the release of chemical platelet factors into blood. Platelet activation causes them to change their shape, allowing them to stick to and build up on vessel walls, forming a dense plug that is held together by fibrin. Fibrinogen binds to the integrin receptors, aIIbb3, on activated platelets, one receptor at each end of the fibrinogen molecule, thereby acting as a bridge to link platelets together in order to bring about platelet aggregation. The mass of fibrin fibrils and platelets can entangle blood cells and plasma, helping to increase the mass of the blood clot at the site of damage.
Other interactions
In addition to forming fibrous clots and acting to aggregate platelets, fibrinogen is involved in other intermolecular interactions through its binding to various proteins and cells. Fibrin can interact with several cell types, including endothelial cells, smooth muscle cells, fibroblasts, leukocytes and keratinocytes. Fibrin can bind to and accumulate the cells required for the inflammatory response and for tissue repair at the site of damage.
In addition, fibrin can bind to several different proteins, including fibronectin, albumin, thrombospondin, von Willebrand factor, fibrulin, fibroblast growth factor-2, vascular endothelial growth factor, interleukin-1. Binding of fibrin to these proteins often results in a change to the structure and properties of the blood clot.
· Fibronectin is found in blood and connective tissue, and is involved in cell adhesion and wound healing. Fibronectin can be cross-linked to fibrin, affecting clot size and density.
· Albumin is a blood protein. Its binding to fibrin affects the lateral aggregation of fibrin, altering the thickness of the fibrin fibre.
· Thrombospondin is released from the alpha-granules of activated platelets, and is involved in platelet aggregation. It acts by stabilising the interactions between fibrin and the integrins receptors on platelets.
· Von Willebrand factor is a blood protein involved in platelet adhesion. It can be cross-linked to fibrin through the action of factor XIIIa, and may be important in forming a fibrin-platelet blood clot.
· Fibrulin is an extracellular matrix protein found in blood that can promote platelet adhesion to the extracellular matrix. Its binding to fibrin may promote platelet adhesion.
· Binding of fibrin to fibroblast growth factor-2, vascular endothelial growth factor, and interleukin-1 helps localise these proteins to the site of tissue damage, in order to promote vascular cell responses needed for wound healing and to repair blood vessels.
Hyaluronic acid and wound healing
Hyaluronic acid (HA) is a component of the wound extracellular matrix. HA binds to fibrin, the fibrin matrix helping to organise a HA matrix, which is important for subsequent wound healing. HA could also have a regulatory role in the control of fibrin degradation and polymerisation, thereby altering the structure of the fibrin polymer gel. The HA-fibrin matrix increases the permeability of the clot and may act as a scaffold through which cells can migrate, either releasing trapped cells or providing passage for incoming cells involved in tissue repair. As cells migrate into the wound for repair, the HA-fibrin matrix breaks down with the help of hyaluronidase and plasmin, becoming replaced by a matrix formed from collagen and glycosaminoglycans. The degradation products of both fibrin and HA are important for the regulation of the inflammatory response and for blood vessel repair.
Fibrinolysis,
Dissolving the Clot
As damaged tissue is repaired, the fibrin clot mass must be dissolved in order to maintain the fluidity of blood. Plasmin degrades fibrin within clots. Plasmin exists in blood as the zymogen plasminogen, which is converted to active plasmin by the proteolytic action of tissue-specific or urokinase-type plasminogen activators. Plasmin cleaves fibrin molecules within a clot at specific lysine residues (see Figure 2, previous page): one in each of the Aa protuberances, and the others within the coiled coil regions of Aa, Bb and g. These cleavage reactions break the molecule into five pieces, two Aa protuberances, two end pieces each containing a D domain, and a central portion containing the E domain. If the fibrin polymer has been cross-linked, then two D domain-containing end fragments from different fibrin molecules will be cross-linked together (see Figure 3, previous page).
The equilibrium between clot formation and degradation is a sensitive one that must be tightly regulated – a weak clot could result in a haemorrhage, while a strong could lead to thrombosis and blockage. Various proteins can bind fibrin and regulate the amount of clotting, cross-linking, and fibrinolysis. These include:
Clotting
· Fibrin can bind thrombin at a separate, non-substrate site in order to limit its diffusion and, therefore, its ability to propagate clotting.
· Fibrin can bind anti-thrombin I, down-regulating the amount of thrombin in clotting blood.
Cross-linking
· Fibrin can bind Factor XIII and regulate its activation to Factor XIIIa, thereby suppressing fibrin polymer cross-linking.
Fibrinolysis
· Fibrin can bind a2-anti-plasmin, plasminogen activator inhibitor-2, and plasminogen to regulate the initiation and propagation of fibrinolysis.
Next: Fibrin and Cancer
Previous: Fibrinogen,
Finishing the Coagulation Cascade