DNA Ligase IV
|
|
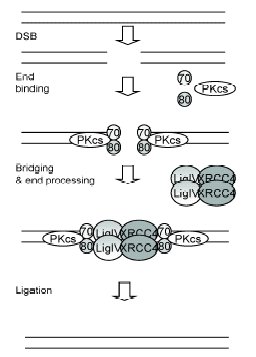
Repair of DNA DSBs by NHEJ in mammalian cells. PKcs is DNA-PKcs, 70 is Ku70, 80 is Ku80, LigIV is DNA
ligase IV, XRCC4 is XRCC4 Reprinted form Acta Biochimica Polonica 50(4), E. Pastwa and J. Blasiak, Non-homologous DNA End Joining, 891-908, 2003, PMID: 14739985. |
NHEJ, How It Works
Non-homologous end joining (NHEJ) functions in all kinds of cells, from bacteria to man, and is involved in many different processes, such as DNA repair, telomere maintenance, and the insertion into the genome of HIV-1 and repetitive sequences. NHEJ of double-stranded breaks (DSBs) in DNA is accomplished by a series of proteins that work together to carry out the synapsis, preparation and ligation of the broken DNA ends. The main proteins involved in NHEJ in eukaryotes are DNA ligase IV, XRCC4, the catalytic subunit of DNA-dependent protein kinase (DNA-PKcs), Ku protein, and possibly Artemis. Deficiencies in any one of these proteins results in hypersensitivity to DNA DSB-inducing agents, such as ionising radiation.
Three steps have been identified in the NHEJ process: DNA end-binding and bridging, terminal end processing, and ligation.
(i) DNA End-binding and Bridging
NHEJ is initiated by the recognition and binding of the Ku protein to the broken DNA ends; Ku is a heterodimer of Ku70 and Ku80 that forms the DNA-binding component of DNA-dependent protein kinase (DNA-PK). Ku forms a ring that encircles duplex DNA, cradling two full turns of the DNA molecule. By forming a bridge between the broken DNA ends, Ku acts to structurally support and align the DNA ends, to protect them from degradation, and to prevent promiscuous binding to unbroken DNA. Ku effectively aligns the DNA, while still allowing access of polymerases, nucleases and ligases to the broken DNA ends to promote end joining.
Once Ku is in place, it recruits the catalytic subunit of DNA-PK, namely DNA-PKcs, which is a serine/threonine kinase in the phosphoinositide 3-kinase (PI3K) family that is believed to be a signalling molecule in response to cellular stress. DNA-PKcs can phosphorylate several nuclear proteins in vitro, including DNA ligase IV and XRCC4. The phosphorylation of DNA ligase IV and/or XRCC4 may affect their interactions with Ku and other proteins.
(ii) Terminal End Processing
NHEJ requires two DNA blunt ends in order to join them together. In some cases, terminal processing of the DNA ends is required before ligation can occur. For instance, a single-stranded overhang requires DNA synthesis by a polymerase to fill-in gaps, which creates blunt ends that can be ligated. Alternatively, single-stranded overhangs can be trimmed off via nuclease activity, a role that may involve the Artemis protein, which belongs to the metallo-beta-lactamase family (IPR011084). DNA-PKcs phosphorylates and binds to the Artemis protein, and the resulting Artemis/DNA-PKcs complex is thought to have nuclease activity that cleaves 5’ and 3’ DNA overhangs.
(iii) Ligation
Once the blunt ends are in place, the XRCC4/DNA ligase IV ligation complex is recruited to join the DNA ends together. DNA ligase IV carries out the ligation step, but it requires the binding of XRCC4 to do so. XRCC4 functions as a regulatory element to stabilise DNA ligase IV, to stimulate ligase activity, and to direct the ligase to the site of DNA breaks via its recognition helix and DNA-binding capacity.
Numerous studies have been directed to identifying other component of the NHEJ system.